IRB Support – Ethical, Thorough, and Approval-Ready
The IRB process can feel overwhelming, especially when you’re balancing ethical requirements with the pressure to move your research forward. As you work to ensure participant protection and meet university standards, the details and documentation required can quickly become daunting. But you don’t have to handle this alone. Our team is here to help you navigate each step, making sure your research meets all ethical guidelines and is prepared for swift approval.
- Customized for Your Study and Requirements: Your research deserves an approach tailored specifically to its unique needs. We customize our IRB support to fit the ethical considerations, goals, and requirements of your university. Our experts understand institutional standards and will help you craft an application that demonstrates a strong commitment to participant protection and ethical compliance.
- Unlimited Revisions Until Approval: Dealing with repeated IRB feedback? We’re committed to working with you until your application is approved. Our team addresses each round of feedback promptly and precisely, ensuring every detail meets the standards required for approval—at no additional cost.
- Fast, Reliable Turnaround: With tight deadlines, you need fast, efficient support that doesn’t compromise quality. Our consultants deliver results quickly and can expedite further if needed, so you can keep your research on track and move forward confidently.
Recognized as an Inc. 500 company, Precision Consulting has nearly two decades of experience supporting doctoral candidates. We’re here to make the IRB process manageable and ensure your research is positioned for success—ethically and effectively.
IRB – Precision Consulting
even on larger
projects
Don’t Let the IRB Process Slow You Down – Call Us Today for Expert Guidance!
The IRB process can feel overwhelming, with its layers of ethical considerations and detailed requirements designed to safeguard participants. For many doctoral candidates, tackling this stage alone is daunting and time-consuming. We’re here to help. Our team ensures that every step is clear and manageable, so you can meet your university’s standards while focusing on what matters most—your research.
Your research deserves the right guidance. Call us (646) 553-4730 for one-on-one support tailored to your needs. Whether you’re refining your ethical framework or finalizing your IRB submission, we’re ready to help you move forward smoothly and effectively.
There are 3 ways to initiate contact with us:
- Please review and submit the following form. Someone from our team will contact you within 1 hour (during business hours), or at your requested time.
- Our consulting team is available via telephone Monday through Saturday from 8:00 A.M. to 8:00 P.M Eastern Time. Feel free to call us on (646) 553-4730!
- We pride ourselves on our very prompt and in-depth e-mail responses, 365 days per year. We normally answer all urgent queries very promptly, including late-night and weekend requests. You can email us at Info@PrecisionConsultingCompany.com
Please be prepared to discuss the specifics of your project, your timeline for assistance, and any other relevant information regarding your proposed consultation. We respect the confidentiality of your project and will, at your request, supply you with a Non-Disclosure Agreement before discussing specifics.
Protecting Your Participants with Confidence
Protecting your participants is at the heart of the IRB process, and we’re here to make sure your application reflects that commitment—thorough, ethical, and ready for approval.
Protecting participants from any potential risk is the central focus of the IRB approval process. As a doctoral candidate, you’re responsible for clearly outlining these safeguards, which can feel overwhelming. Fortunately, we’ve guided many clients through this process, and we’re here to ensure your IRB application is comprehensive and ready for approval.
Complete and Accurate IRB Applications
Preparing a full, accurate IRB application is essential to avoid delays and gain approval. We walk you through each section, ensuring your application covers all necessary details—especially those related to participant safety, anonymity, and confidentiality. With our guidance, you can feel assured that your application reflects the high level of ethical consideration expected by the IRB.
Designing Ethical Research Protocols
Whether your study is qualitative or quantitative, clear, ethical research protocols are key. We work with you to create protocols for each phase, from informed consent to data collection and handling. If unexpected situations arise during data collection, you’ll be prepared to address them responsibly and in alignment with IRB standards.
Ready for IRB Approval?
You don’t have to face the IRB process alone. With our expertise and personalized support, your IRB application will demonstrate thorough planning and a commitment to participant protection—moving you one step closer to approval and research success.
Call us today (646) 553-4730 for a free consultation, and let’s make sure your IRB application is ready for success.
Comprehensive IRB Support for a Smooth Approval Process
Getting IRB approval involves more than just filling out forms—it requires thorough planning, detailed ethical protocols, and specific documentation tailored to your study. With our comprehensive support, every part of your IRB application reflects a high standard of ethical care, so you can move forward with confidence.
Customized Informed Consent Forms and Supplemental Materials
Your informed consent forms need to be as unique as your study—clear, specific, and fully compliant with IRB standards.
Creating informed consent forms tailored to your data collection methods is crucial for approval. We help you develop these forms and other supplemental materials specific to your methodology. With everything designed to align with your research approach, you can get IRB approval faster and begin data collection sooner.
Starting with a Methodological Review
Avoid costly revisions later by refining your research design now.
If you’re early in the process, starting with a methodological review can save time and ensure smooth IRB approval. We work with you to review and refine every detail of your research plan, including:
- Defining your study’s topic, purpose, and design
- Clarifying research questions and hypotheses
- Evaluating instrumentation and discussing validity and reliability
- Selecting the most appropriate analytic methods
By honing your methodology at this stage, you’ll be ready to submit a well-crafted, IRB-ready proposal.
Focused Support for Approved Proposals
Get your IRB application ready with tailored guidance on ethical compliance.
If you already have an approved proposal and only need IRB application support, we provide detailed guidance on each critical component, including:
- Data collection access and timelines
- Confidentiality protocols and minimizing risk
- Meeting your university’s specific IRB standards
With everything in place, your application will be carefully crafted to meet rigorous ethical and institutional requirements.
Expertise Across Fields and Methodologies
From psychology to nursing, your IRB application will benefit from field-specific expertise.
Whether your study is qualitative, quantitative, or mixed-methods, we support IRB applications across a variety of fields. Our team includes PhD-level experts in psychology, nursing, business, education, and more. With comprehensive guidance, your IRB application will be ready for swift approval, so you can begin data collection as soon as possible.
Ready to Get Started with Your IRB Application?
With our expert guidance, your IRB application will be thorough, ethically sound, and ready for approval. Call us today (646) 553-4730 for a free consultation, and let’s discuss how we can make the IRB process easier for you. Get your research moving forward with confidence!
Our Process for IRB Application Support
Securing IRB approval involves several key steps to ensure that all aspects of your study meet ethical standards. Our process provides structured, comprehensive support to prepare each component of your IRB application thoroughly and accurately. Here’s how we assist you through each step:
Step 1: Crafting IRB Supplementary Materials
Build a foundation of trust with participants through clear, ethical study documentation.
To meet IRB requirements, you’ll need to provide detailed supplementary materials that guide participants through your study and demonstrate your commitment to ethical standards. We help you develop:
- Informed Consent Forms: Tailored to your study design and data collection methods, ensuring participants fully understand their rights and involvement.
- Study Invitations: Clear, welcoming invitations that encourage participation while being transparent about the study’s purpose and scope.
- Interview Protocols: For qualitative studies, structured interview guides that uphold ethical practices while collecting valuable data.
These supplementary materials set a strong ethical foundation, making your application IRB-ready.
Step 2: Developing a Complete IRB Application
Ensure your IRB application is comprehensive, accurate, and fully aligned with institutional standards.
The IRB application itself is central to gaining approval. We guide you through the full process to ensure your application meets university and ethical standards:
- Methodology Summary: Clear explanations of your research design, including your approach (qualitative, quantitative, or mixed-methods) and data collection methods.
- Ethical Protocols: Detailing the steps you’ll take to protect participant confidentiality, minimize risk, and respond ethically to any potential issues during data collection.
- Research Justification: Clearly articulated purpose and significance, outlining the value of your study and how it addresses gaps in the existing literature.
By covering each part of the IRB application thoroughly, you create a well-supported case for approval.
Step 3: Final Review and Submission Preparation
Gain confidence with a polished, approval-ready IRB application.
Before submission, we conduct a final review to ensure every detail aligns with ethical and institutional requirements. This includes:
- Full Document Check: Verifying that all sections, from informed consent to study protocols, are complete and accurately documented.
- Compliance Verification: Ensuring all components meet university-specific guidelines for ethical research.
- Polished, Submission-Ready Application: Final edits and formatting to ensure a professional, cohesive application.
With everything in place, your IRB application will be ready for a smooth, confident submission process.
Ready to Secure IRB Approval?
Our structured, step-by-step support ensures your IRB application is thorough, ethical, and fully prepared for approval. Call us today (646) 553-4730 for a free consultation and let’s get started on making your IRB process seamless and successful. Move forward with confidence, knowing every detail is covered!
Let’s keep it a secret…
Before sharing your materials with us, we will send you our Non-Disclosure Agreement, which guarantees that your work materials, and even your identity as a client, will never be shared with a third party.Hi! In other instructional videos, we talked about development of your topic, research, alignment among your foundational elements, methodology construction, and dissertation editing. All of this is great — the foundation is actually the most important part of your study.
However, it’s not the only necessary part of the dissertation process, and in this video, we’ll talk about the IRB process. It’s easy to forget this, since it’s not part of your dissertation manuscript — if it’s overlooked, though, or even if you start planning before you have your IRB process in mind, then you might hit a brick wall here. Because of the challenges of obtaining IRB approval, we often provide help with dissertation or thesis research at this stage — hopefully this video will help to clarify how to navigate the IRB application process.
Actually, you might be watching this video after your chair or another committee member remarked about the IRB-related difficulties that might follow if you pursue this or that plan in terms of sample or protocol. It doesn’t come up in the process until after the proposal — the first three chapters — at the vast majority of universities.
Some major online universities deviate from this pattern, and I should mention that we have a great deal of experience with those schools and how they operate differently! In either case, though, the IRB process appears only after a great deal of planning and development on your part. Thankfully, then, committees are often thinking about this even when you’re not!
It’s a significant step, as it determines whether you’ll be able to conduct your study in the first place. Without it, you won’t be able to even start recruiting for participants. You’ll be stuck in the dissertation, and without the ability to do more than set up assistance from an organizational partner or plan for recruitment.
And the purpose is to ensure that you’re acting within all ethical bounds as you conduct your research as you take on all those other steps, from recruitment and collection to analysis and data protection, even long after your study has been conducted. This is especially important for your university, as you’ll be acting as a representative of the school in carrying out your research.
Let’s say that you’ve worked with the topic that we first discussed in the problem statement module. Here’s the research gap:
And here it is! To catch you up to speed, you’re doing a phenomenological study! If you need a refresher on what a phenomenological study entails, be sure to check out our Qualitative Methods video. We’ll engage with the important IRB elements for this study, in particular, as it’s a fraught one and allows us to think through many of the issues you might encounter in your study.
First, an IRB overview: In some ways, your IRB application is an extension of your methodology chapter. It relies on your having clear procedures for recruitment, collection, and analysis, for one thing, and it also borrows information from the methodology in your dissertation about ethical protections that you’ll afford to participants, helping to safeguard their anonymity and confidentiality.
I should stop a moment and clarify about how the process looks different for primary and secondary research studies. Primary studies are ones where you interact directly with participants, interviewing them, conducting a focus group, observing them, or administering a survey.
Some of you might have decided to use an outside service, such as SurveyMonkey or Qualtrics, to recruit and collect data. In this case, you’re not having direct contact with participants, but since the data are being generated for your study, it’s still primary.
It’s a secondary study when you’re examining a data set that already exists, even as you plan your research. Most often, this is a quantitative study, as numerical data are much more likely to be found “in the wild.” For a review on what quantitative methods entail, be sure to have a look at our Quant Methods video.
Although completing the IRB application for secondary research studies is typically somewhat less involved than those for primary studies, there is still quite a bit of complexity involved in these applications, as you will undoubtedly come to recognize.
If you feel unsure about how to get started on your IRB application for your dissertation and would like help with this big step in your research, give us a call or send a quick email to ask about how we can help. We’ll definitely be here to do that, and can help with IRB applications no matter how tricky they might be!
With our vast experience providing this assistance with thesis and dissertation research, especially from the major online universities, we often help to navigate this rather complicated and lengthy process. And, although our specialty is crafting IRB applications to support qualitative research, we also have vast experience with completing these documents for all types of quantitative thesis and dissertation projects.
As this discussion will soon highlight, having this sort of comfortable familiarity with your methodology will definitely pay off when it comes time to fill out these IRB forms!
And, when you see all of the possible pitfalls associated with filling out these documents, you’ll see how awesome it is that we include unlimited revisions for this work. But, I’m getting ahead of myself!
Okay, back to the IRB application, and what this distinction means for your IRB is that secondary studies are much more straightforward to get to IRB approval — you’re not recruiting or collecting data from participants, and it’s just the access to the data set (if not publicly available) that you’ll need to demonstrate.
For primary research studies, though, issues abound, and trouble lurks around each corner.
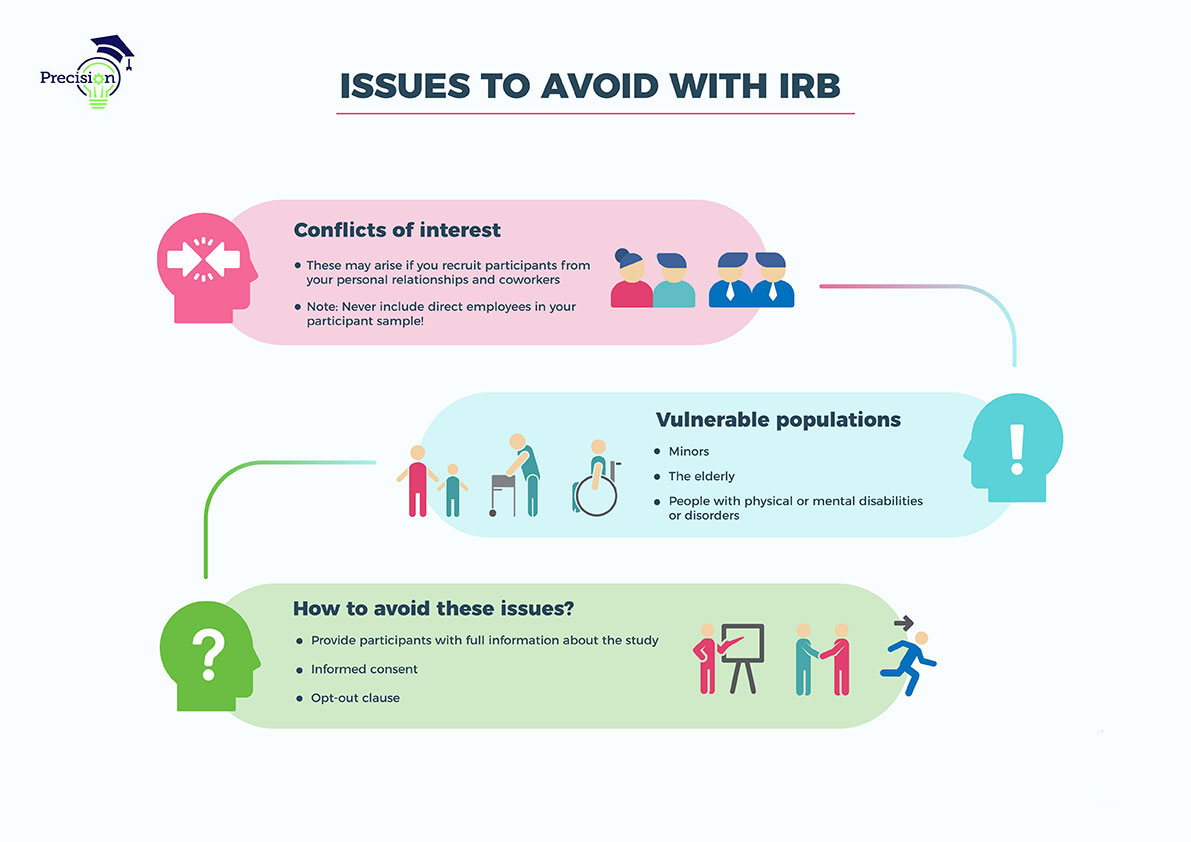
I have a table here that will help walk us through it! First up is eliminating conflicts of interest. Unfortunately, there are about as many potential conflicts as there are interests! Thankfully, they cluster in certain areas, particularly for doctoral candidates, and targeted dissertation help allows you to get through them. Without incredibly large budgets, candidates are often left sampling from around them. Within education, it might be teachers that you know or work with, or perhaps that you manage. It might be middle managers in your company or in a colleague’s company. It might be nurses in your unit. In all of these cases, your relationship with the (potential) participants isn’t simply a researcher-participant one.
Often, this is permissible, but problems arise when you manage or are in some way in charge of potential participants, and often no IRB protections can help to overcome this conflict for your dissertation. In most other situations, though, and even in the last one, there are options that can help.
For this study again, we’re working with a group of nurses and other mental health care workers. Let’s say that, for this study, you work at the facility as an intern as you complete your doctoral degree. That’s common! In this case, your relationship goes outside the bounds of just researcher-participant. Right, you work with them. However, you’re by no means a superior. As a result, they aren’t likely to feel as though there is any cost in participating. There is the chance that confidentiality won’t be observed, and that you’ll report the content from their interview to colleagues or to a superior.
Problems, then, might be your dual relationship or potential disclosure of information. For this reason, it’s important to provide potential participants with protections. Often, these are done in the informed consent form, where the participant is apprised of the benefits, if any, and the ways that you’ll ensure that ethical boundaries are enforced. In the informed consent form, it will be necessary to make clear that participants can opt out of the study at any time, should they feel uncomfortable or for any other reason. In addition, they should be notified that all information they will provide may be viewed by you and perhaps by your chairperson, from here identifying all the ways that their information will be protected from intentional or accidental revelation…
…Specifically, through an opt-out clause, confidentiality guarantee, or both.
If you’re struggling with a conflict of interest of this kind, please let us know! We’re so happy to provide the help needed to help you move forward and without having to reconceptualize your dissertation research.
Now if you were to be a superior, further protections would be needed, as the other relationship turns those participants into a vulnerable population — they might feel pressure to participate, that there will be costs if they don’t, and also that there might be costs if they do participate and say the wrong thing.
More traditionally, though, people such as minors, the elderly/infirm, and people with some form of physical or mental disability or disorder are considered vulnerable, irrespective of any additional relationships with the researcher. This is because they “depend on” others in some sense, and without due care, can be or feel taken advantage of or under duress. In our consulting for the dissertation, we often see clients who may initially want to sample from a population of elementary, middle, or high school students, and this can often be difficult for this reason.
Within psychology and nursing, working with patients in some way can also be difficult because of the vulnerability inherent in being a patient — whether suffering from mental illness of contending with a physical ailment. In these cases, getting “distance” from the participants can often be helpful, and one way to do this is to interview clinicians who work with those patients or use anonymous secondary data, if available. The right participants for a dissertation help to make approval a great deal easier.
To apply this to our study, no traditionally vulnerable populations are being sampled from, at least not by design. Instead, it might be that pregnant women are included in the sample. And again, the ability to opt out and protection from disclosure of any information provided as part of the study or the publication of your dissertation would be helpful protections offered here.
So again here, problems might be the pressure to participate and the costs of with participation and non-participation, and solutions might be that opt out clause and a confidentiality guarantee.
Next is risk. According to the landmark Belmont Report (1979), by the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research (that’s a mouthful!), all participants are, by definition, exposed to at least minimal risk. It’s moving beyond this that we should worry about — it’s those kinds of “expected risks” that apply in a given situation and with given participants. Estimating the risks to participants can be tricky, and a consultant can shed light on the many possible issues for your dissertation. Such risk might involve psychological stress that exceeds everyday stress that people encounter. It might extend to unintended disclosure of confidential information, like educational or medical records, and it might be the perceived pressure to participate in itself — such as when the participant is a subordinate of the researcher.
So again here, risks abound here: financial, occupational, and psychological.
Again, that’s not the case here with our study, but we are asking nurses and other mental health care workers to engage with recalled stress — this might involve psychological stress that exceeds what they might encounter in their day-to-day lives. Actually, they might recall trauma in participating, particularly in the interviews normally part of qualitative research, and it will be critical to protect them from that.
Again, just like with our other protections, the ability to stop participating at any time, even in the middle of the interview, will be really important, as well the guarantee of confidentiality. However, because the study exposes participants to significant risk by design, more protection might be required.
So in addition to the very standard opt out clause and confidentiality guarantee, there is something called an interview break. Specifically, the researcher should be attuned to gestures or other non-verbal cues that signal distress and, when seeing them, offer to the participant to stop or take a break.
In addition, the researcher should not provide counseling services him or herself, and particularly not during the interview, but the researcher can provide the participant with one or more counseling resources to take advantage of.
Finally, the researcher can offer to debrief with the participant after the interview, allowing them to ask questions and so the researcher can share more information about the purpose of the study and other issues that might be germane to participation.
Once people do agree to participate, one it’s necessary to make sure that their information will be anonymous — both when reported in the dissertation manuscript and when the researcher engages with third parties, such as a statistician or consultant, about that research. The same applies for confidentiality — information other than what is needed should not be reported, and all information, regardless of relevance, will be protected from being revealed, on purpose or on accident, and in all its forms, both physical and digital.
Let’s apply these to our study. Again, we’ll be interviewing mental health workers, and we’re interviewing them about their experiences of victimization and their decisions regarding reporting. The interviews will be recorded by a program on a personal computer, transcribed via an online service, and then analyzed using NVivo.
So that means third party assistance and computer vulnerability are potential problems here.
For this study, then, protections will need to be in place first on the computer — it should be password-protected, and the researcher should be the only person with access to that password. The informed consent should note that a third party will be utilized to transcribe, and all records should be de-identified prior to that transcription. I think you can see now that the informed consent is an important document for your dissertation! Before you submit it with your IRB application, consulting with a professional about its contents and language can be really helpful.
I hope this has been helpful! It does help with the work of considering more fully some of the issues and concerns that are part of the IRB application process, particularly those ethical issues that often arise in the course of planning for even a normal study!
Again, we did refer to at least one form — the informed consent form — that should be submitted in addition to the IRB application here. Since the methodology and IRB application should, together, provide enough information that anyone else can complete your study for you, it’s necessary to also include the following:
Completing the many different documents necessary to secure IRB approval with your university can be an extremely challenging process, and it is easy to overlook required forms or specific content required to obtain approval.
Of course, submitting an incomplete or improperly completed IRB application packet can result in significant delays in your study, as this requires that you revise and resubmit various documents in your application often repeatedly before being allowed to move forward to data collection.
To prevent this headache during an already long dissertation process, many of our clients reach out before their IRB application phase to receive help with this process from the start.
We can assist with completing the IRB packets for thesis and doctoral candidates from any university, although we have particularly extensive experience with the major online universities’ application packets.
Again, we can help with your IRB application itself, your informed consent forms, your CITI or NIH certificates, recruitment materials, and permissions from all the sites that you might be partnering with in your research. We can even help with editing for APA style — a requirement that doesn’t go away here!
And remember, as I was saying earlier, our assistance will involve unlimited revisions with no extra charge as needed to make sure that you receive approval at any stage of your dissertation. Even the pickiest of IRBs — and we’ve had experience with the pickiest of them — you can be assured of getting past this hurdle successfully!
Then you’ll be on to your data collection and the rest of your study! Thanks for watching!