Institutional Review Board Consulting Services
Capability
Application Process
Institutional Review Boards (IRBs) are crucial entities that oversee and approve research studies involving human subjects. The IRB is responsible for ensuring that research is conducted in an ethical and safe manner, with the protection of participants' rights and welfare being of the utmost importance. At Precision, we offer a range of valuable services to ensure that researchers conducting human subjects research are following ethical guidelines and regulations. When we work with clients, we take the time to explain the purpose and importance of the IRB process, as well as the specific requirements that must be met in order for research to be approved.
We know that IRB application should be taken seriously as without securing an approval, no data collection can commence in any form and could result in legal ramifications if ignored. Since 2006, our in-house IRB consultants have worked directly with the businesses and research institutes that we collaborate with, offering services that will help them navigate the IRB application procedure. Our team of IRB advisors has produced numerous successful IRB applications across a variety of disciplines, including social marketing, sociology, psychology, medicine, and information technology, to name a few. These applications ranged from research and exemption determination to creating recruiting and data collection materials to annual reviews.
There are 3 ways to initiate contact with us:
- Please review and submit the following form. Someone from our team will contact you within 1 hour (during business hours), or at your requested time.
- Our consulting team is available via telephone Monday through Saturday from 8:00 A.M. to 8:00 P.M Eastern Time. Feel free to call us on (702) 708-1411!
- We also pride ourselves on our very prompt and in-depth e-mail responses, 365 days per year. We normally answer all urgent queries very promptly, including late-night and weekend requests. You can email us at Info@PrecisionConsultingCompany.com
Please be prepared to discuss the specifics of your project, your timeline for assistance, and any other relevant information regarding your proposed consultation. We respect the confidentiality of your project and will, at your request, supply you with a Non-Disclosure Agreement before discussing specifics.
One of the key services we provide is assisting with the development of research protocols. We work closely with researchers to ensure that their protocols are clear, comprehensive, and in compliance with all relevant regulations. This includes reviewing research proposals, identifying potential risks to participants, and helping researchers develop strategies to mitigate those risks.
We also provide guidance and support throughout the IRB review process. This may involve preparing and submitting the necessary documentation, communicating with IRB members and staff, and addressing any concerns or questions that arise during the review process. Our expertise and experience in navigating the IRB system can help ensure that the review process goes as smoothly and efficiently as possible.
Additionally, we provide training and education to researchers, coordinators, and other stakeholders involved in human subjects research. This may include workshops, webinars, or one-on-one consultations to help ensure that everyone involved in the research process understands their roles and responsibilities, as well as the ethical considerations that must be taken into account.
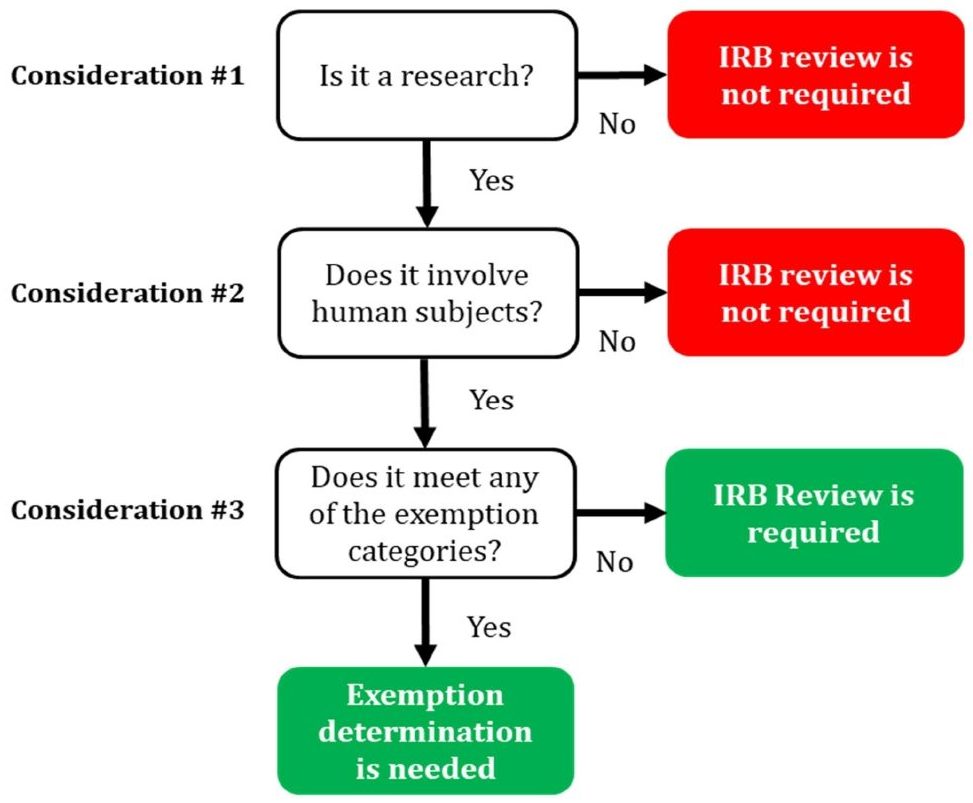
The IRB process can undoubtedly appear difficult and overwhelming, which is why Precision's IRB consultants are available to assist you in completing it successfully. First-timers frequently don't know if they require IRB clearance for the project they are about to start. So if you are a first-timer IRB applicant or at a loss on whether or not you need an IRB approval, we are here to help you. We have created an easy-to-understand IRB decision tree that can help a layperson decide whether or not an IRB review is necessary for their study.
As IRB service providers, we understand that we play a critical role in helping to ensure that human subjects research is conducted in a safe, ethical, and responsible manner. Our knowledge, expertise, and commitment to the highest ethical standards help to protect the rights and welfare of research participants, while also advancing the scientific knowledge and understanding of the world around us.
Do not fret even if you do now even know where to begin, as our well-trained, well-experienced IRB advisors are here to support you in any needs or issues that you may have. Our team of IRB advisors are able help in the following areas, but not limited to:
- Research determination
- Exemption determination
- Expedited reviews
- Full-Board reviews
- Development of recruiting and data collection materials
- Amendments and updates
- Annual (Continuing) Review
Gained from more than 10 years’ worth of experience, here are some tips that we think would be helpful to keep in mind when doing your IRB application:
Plan ahead, Start early. Applications are reviewed on a first-come, first-served basis. Initial feedback from the IRB could take 3-6 weeks and final approvals usually do not come until 2-3 rounds of review. IRB does not entertain rush requests and therefore it is the responsibility of the proponents to submit their application as early as possible to avoid delays in their research. We recommend starting preparing your IRB application at least 8 months from the intended start date of your research.
Be thorough, carefully read instructions. Any type of IRB application comes with a set of detailed instructions that should be diligently followed when completing the application. Based on our experiences, your best shot in minimizing revisions on your application is to follow every instruction and provide answers to each of the components in the application. It is always better to do things right first time as revisions take a lot of time. Remember that putting extra time and care into your application at the start of the process can significantly reduce the amount of time the entire process will take.
Attend IRB trainings. Attend IRB trainings and workshops to familiarize yourself with the IRB requirements and guidelines. This will help you to better understand the IRB process and prepare a more effective protocol.
Utilize IRB resources. Take advantage of the resources provided by the IRB, such as guidance documents and sample protocols, to help you develop a well-written and thorough protocol.
Seek assistance. Consult with the IRB staff or independent IRB services providers such as Precision Consulting to clarify any questions you may have about the IRB process, protocol development, or specific requirements.
Be thorough and responsive. Ensure that your protocol is complete and contains all necessary information. This includes a clear description of the study design, recruitment methods, informed consent process, risk and benefits to participants, data analysis plan, and confidentiality measures. Respond promptly to any requests for clarification or additional information from the IRB staff. This will help to expedite the review process and minimize delays.
Stay organized. Keep track of all correspondence and documents related to the IRB process. This will help you to stay organized and ensure that you are meeting all IRB requirements and deadlines.
Previous IRB projects include:
Research Area | IRB Review Type | Research Method | ||
|---|---|---|---|---|
Public Health | Expedited | Quantitative correlational | Investigate the role of socio-economic status in relation to sexually transmitted infections testing behaviors and condom usage among the nonsurvival sex worker population | IRB application, HIPAA authorization form, recruitment materials, data collection protocol, survey |
Education | Expedited | Mixed methods | Examine the impact of an institutionalized diversity plan, including the perceived sense of academic achievement, sense of belonging, and program completion among African American students in a community colleges | IRB application, informed consent, recruitment script |
Social work | Full Review | Qualitative hermeneutic phenomenological | Explore the lived experiences of African American Youth with adverse childhood experiences in the context of COVID-19 | Interview protocol, recruitment email, IRB application |
Information Technology | Expedited Review | Qualitative descriptive | Explore the use of servant leadership by community-based organizational leaders from the CEO perspective | Letters of cooperation, IRB application, interview guide, questionnaire, informed consent |
Pharmaceutical | Exempt Review | Quantitative descriptive | Determine the extent of pharmaceutical counterfeiting in Florida | IRB application, research protocol, and data analytics sheet |
Public Policy | Full Review | Qualitative case study | Explore the risk and resilience of federal agents in a US federal government agency | Permission letter to federal agency, IRB application, informed consent, recruitment materials, semi-structured interview protocol |
Finance | Expedited Review | Qualitative descriptive | Determine the role of the financial robo-advisor (FRA) will play in providing advice to Gen Z consumers | IRB application, informed consent, semi-structured interview protocol, social media permission letters, recruitment materials |